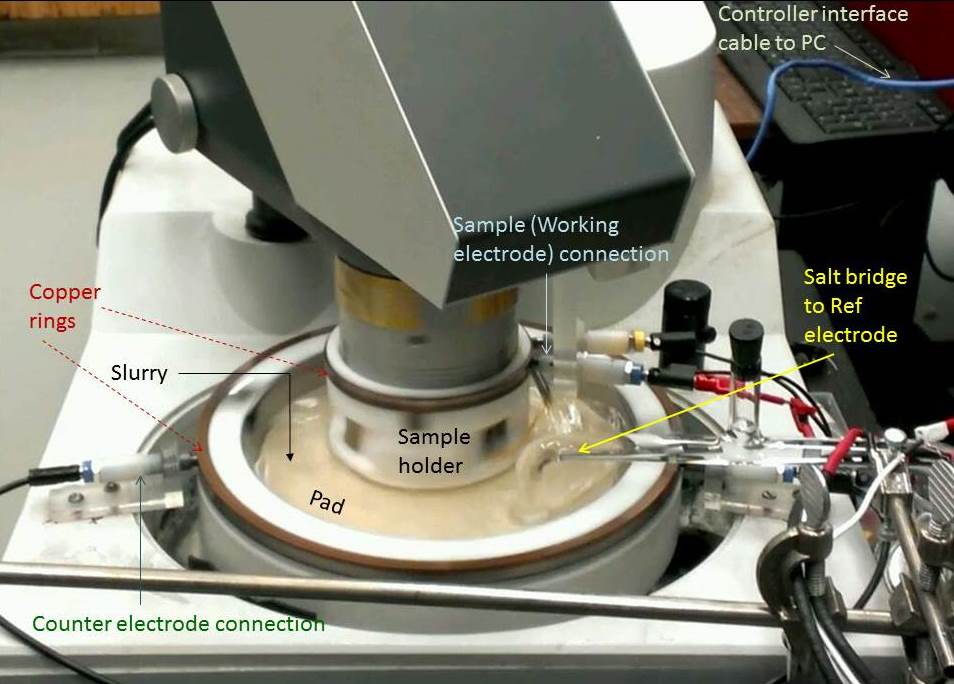
Tribo-Electrochemical Setup for Metal CMP utilizes controlled "corrosion-like" surface modifications to (electro)chemically form structurally weak surface complexes that can be removed with minimal mechanical abrasion. To support this function, CMP slurries for metal components usually contain a pH-adjusted aqueous background solution, an oxidizer, and a complexing agent and/or a "corrosion inhibitor". Slurry-stabilizers like surfactants are also used to prevent coagulation of abrasive particles in the electrolyte, which helps to minimize "scratches" and other defects caused by large particle aggregates. Strategic selections and combinations of these slurry consumable materials dictate the overall performance of metal CMP. At the metal-slurry interface, the CMP-enabling corrosion reactions tend to occur in the mixed potential mode, and thus, are predominantly electrochemical by nature. Due to this reason, electrochemical techniques are ideally suited for laboratory-scale investigations of such reactions. However, since they occur in the presence of surface abrasion during CMP, tribology plays a substantial role in governing the detailed mechanisms and the CMP outcomes of these reactions. In fact, the findings about slurry chemistries can be very different between measurements performed with and without mechanical abrasion/polishing of the CMP surface. Therefore, for comprehensive assessments of metal CMP systems, it is necessary to combine tribology with electrochemical measurements. Part of the tribo-electrochemical setup used in our laboratory for this purpose is shown in the figure below.
The CMP sample, used as a working electrode (WE) for tribo-electrochemical measurements, is housed in a custom-designed Teflon holder. This holder is mounted on to the polishing head (upper moving part) of a commercial (Struers) benchtop polisher. A polishing pad, chosen according to slurry/sample selection, is sized to be affixed to the floor of the Teflon test cell. The floor of the cell represents the polishing platen for CMP, as it is attached to the rotary table (lower moving part) of the polisher. The directions and speeds of both the platen and the sample holder are controlled from a computer using LabVIEW codes developed by our group. The WE sample is pressed down onto the pad, typically at ~2 psi down-pressure, by using the polisher's manual adjustments. The manually set polish-pressure can be independently verified using a pressure sensor under stationary conditions. Published papers that have discussed the tribo-cell shown above: 1. D. Roy, "Electrochemical Assessment of Slurry Formulations for Chemical Mechanical Planarization of Metals: Trends, Benefits and Challenges", ECS Journal of Solid State Science and Technology 7 (2018) P209-P212. Invited Perspective Article; *Open access* 2. C. A. Johnson, S. Wei and D. Roy, "An Alkaline Slurry Design for Co-Cu CMP Systems Evaluated in the Tribo-Electrochemical Approach", ECS Journal of Solid State Science and Technology 7 (2018) P38-P49. 3. M. C. Turk, M. J. Walters and D. Roy, "Tribo-electrochemical investigation of a slurry composition to reduce dissolution and galvanic corrosion during chemical mechanical planarization of Cu-Ru interconnects", Materials Chemistry and Physics 201 (2017) 271-288. 4. M. C. Turk, M. J. Walters and D. Roy, "Experimental considerations for using electrochemical impedance spectroscopy to study chemical mechanical planarization systems", Electrochimica Acta 224 (2017) 355-368. 5. M. C. Turk, X. Shi, D. A. J. Gonyer and D. Roy, "Chemical and Mechanical Aspects
of a Co-Cu Planarization Scheme Based on an Alkaline Slurry
Formulation", ECS Journal of Solid State Science and Technology
5 (2016) P88-P99. 6. X. Shi, D. E. Simpson and D. Roy,
"Tribo-Electrochemical Characterization of Ru,
Ta and Cu CMP Systems Using Percarbonate Based
Solutions", ECS Journal of Solid State Science and Technology 4
(2015) P5058-P5067.
*Appeared Open Access in: JSS Focus Issue on Chemical
Mechanical Planarization: Advanced Material and Consumable Challenges.* 7. C. A. Johnson and D. Roy, "In Situ Electrochemical
Evaluation of Post-CMP Cleaning Reactions for Cobalt and Copper Films under
Brushing Conditions"* ECS Journal of Solid State Science and
Technology 8 (2019) P3163-P3174. *Editor's Choice feature
article (Open Access) in JSS Focus
Issue on CMP for Sub-10 nm Technologies. 8. M. C. Turk, D. E. Simpson and D. Roy,
"Examination of Salicylaldehyde as a Surface
Modifier of Manganese for Application in Chemical Mechanical
Planarization", ECS Journal of Solid State Science and Technology
2 (2013) P498-P505. 10. X. Shi, S.E. Rock, M.C. Turk and D. Roy, "Minimizing the effects of galvanic corrosion during chemical mechanical planarization of aluminum in moderately acidic slurry solutions", Materials Chemistry and Physics 136 (2012) 1027-1037. 11. S.E. Rock, D.J. Crain, C.M. Pettit and D. Roy, "Surface-complex films of guanidine on tantalum nitride electrochemically characterized for applications in chemical mechanical planarization", Thin Solid Films 520 (2012) 2892-2900. 12. S.E. Rock, D.J. Crain, J.P. Zheng, C.M. Pettit, D. Roy, "Electrochemical investigation of the surface-modifying roles of guanidine carbonate in chemical mechanical planarization of tantalum", Materials Chemistry and Physics 129 (2011) 1159- 1170. 13. C. M. Sulyma, C. M. Pettit, C. V. V. S. Surisetty, S. V. Babu and D. Roy, "Electrochemical investigation of the roles of oxyanions in chemical–mechanical planarization of tantalum and tantalum nitride", Journal of Applied Electrochemistry, 41 (2011) 561–576. 14. B. C. Peethala, D. Roy, and S. V. Babu, "Controlling the Galvanic Corrosion of Copper during Chemical Mechanical Planarization of Ruthenium Barrier Films", Electrochemical and Solid-State Lett., 14 (2011) H306-H310. 15. C. M. Sulyma and D. Roy, "Voltammetric current oscillations due to general and pitting corrosion of tantalum: Implications for electrochemical-mechanical planarization", Corrosion Science 52 (2010) 3086-3098. 16. C. M. Sulyma
and D. Roy, "Electrochemical characterization of surface complexes
formed on Cu and Ta in succinic acid based solutions used for chemical
mechanical planarization", Applied Surface Science 256
(2010) 2583-2595.
18. C. V. V. S. Surisetty, B. C. Peethala, D. Roy, and S. V. Babu, "Utility of oxy-anions for selective low pressure polishing of Cu and Ta in chemical mechanical planarization", Electrochemical and Solid-State Letters 13 (2010) H244-H247. 19. S. V. S. B. Janjam, B. C. Peethala, D. Roy and S. V. Babu, "Chemical mechanical planarization of TaN wafers using oxalic and tartaric acid based slurries", Electrochemical and Solid-State Letters 13 (2010) H1-H4. 20. J.P. Zheng and D. Roy, "Electrochemical examination of surface films formed during chemical mechanical planarization of copper in acetic acid and dodecyl sulfate solutions", Thin Solid Films 517 (2009) 4587-4592. 21. C.M. Sulyma,
P.C. Goonetilleke and D. Roy, "Analysis of
current transients for voltage pulse-modulated surface processing:
Application to anodic electro-dissolution of copper for electrochemical
mechanical planarization", Journal of Materials Processing Technology
209 (2009) 1189-1198. 23. J. P. Zheng, B. K. Klug, and D. Roy, "Electrochemical Investigation of Surface Reactions for Chemical Mechanical Planarization of Tantalum in Oxalic Acid Solutions", Journal of The Electrochemical Society 155 (2008) H341-H350. 24. P.C. Goonetilleke
and D. Roy, "Relative roles of acetic acid, dodecyl sulfate and benzotriazole in chemical mechanical and electrochemical
mechanical planarization of copper", Applied Surface Science 254
(2008)
2696-2707. 26. C. V. V. S. Surisetty,
P. C. Goonetilleke, D. Roy, and S. V. Babu, "Dissolution Inhibition in Cu-CMP Using
Dodecyl-Benzene-Sulfonic Acid Surfactant with Oxalic Acid and Glycine as
Complexing Agents", Journal of The Electrochemical Society 155
(2008) H971-H980.
28. S. V. S. B. Janjam, C. V. V. S. Surisetty, S. Pandija, D. Roy, and S. V. Babu, "Oxalic-Acid-Based Slurries with Tunable Selectivity for Copper and Tantalum Removal in CMP", Electrochemical and Solid-State Letters 11 (2008) H66-H69. 29. P.C. Goonetilleke and D. Roy, "Voltage pulse-modulated electrochemical removal of copper surface layers using citric acid as a complexing agent", Materials Letters 61 (2007) 380-383. 30. Y. Hong, V. K. Devarapalli, D. Roy, and S. V. Babu, "Synergistic Roles of Dodecyl Sulfate and Benzotriazole in Enhancing the Efficiency of CMP of Copper", Journal of the Electrochemical Society 154 (2007) H 444-H 453. 31. S. Pandija, D. Roy and S.V. Babu, "Chemical mechanical planarization of copper using abrasive-free solutions of oxalic acid and hydrogen peroxide", Materials Chemistry and Physics 102 (2007) 144-151. 32. S. Ramakrishnan, S.V.S.B. Janjam, U.B. Patri, D. Roy, and S.V. Babu, "Comparison of dicarboxylic acids as complexing agents for abrasive-free chemical mechanical planarization of copper", Microelectronic Engineering 84 (2007) 80-86. 33. K.A. Assiongbon,
S.B. Emery, V.R.K. Gorantla, S.V. Babu and D. Roy, "Electrochemical impedance
characteristics of Ta/Cu contact regions in polishing slurries used for
chemical mechanical planarization of Ta and Cu: considerations of galvanic
corrosion", Corrosion Science 48 ( 34. C.M. Pettit and D.
Roy, "Role of iodate ions in chemical mechanical and
electrochemical mechanical planarization of Ta investigated using
time-resolved impedance spectroscopy", Materials Letters 59
35. P.C. Goonetilleke and D. Roy, "Electrochemical-mechanical planarization of copper: Effects of chemical additives on voltage controlled removal of surface layers in electrolytes", Materials Chemistry and Physics 94 (2005) 388-400. 36. S. B. Emery, J. L. Hubbley, M. A. Darling and D. Roy, "Chemical factors for chemical mechanical and electrochemical mechanical planarization of silver examined using potentiodynamic and impedance measurements", Materials Chemistry and Physics, 89 (2005) 345-353. 37. Y. Hong, U. B. Patri, S. Ramakrishnan, D. Roy and S.V. Babu, "Utility of dodecyl sulfate surfactants as dissolution inhibitors in chemical mechanical planarization of copper", Journal of Materials Research 20 (2005) 3413-3424. 38. P. C. Goonetilleke, S. V. Babu, and D. Roy , "Voltage-Induced Material Removal for Electrochemical Mechanical Planarization of Copper in Electrolytes Containing NO3-, Glycine, and H2O2", Electrochemical and Solid-State Letters 8 (2005) G190-193. 39. Y. Hong, D. Roy and S. V. Babu, "Ammonium Dodecyl Sulfate as a Potential Corrosion Inhibitor Surfactant for Electrochemical Mechanical Planarization of Copper", Electrochemical and Solid-State Letters 8 (2005) G297-G300. 40. V. R. K. Gorantla, K. A. Assiongbon, S. V. Babu, D. Roy, "Citric acid as a complexing agent in chemical-mechanical planarization of copper: Investigation of surface reactions using impedance spectroscopy", Journal of the Electrochemical Society 152 (2005) G404 - G410. 41. V. R. K. Gorantla, S. B. Emery, S. Pandija, S.V. Babu and D. Roy, "Chemical effects in chemical mechanical planarization of TaN: Investigation of surface reactions in a peroxide-based alkaline slurry using Fourier transform impedance spectroscopy", Materials Letters 59 (2005) 690-693. 42. J. Lu, J. E. Garland, C. M. Pettit, S. V.
Babu, and D. Roy, "Relative Roles of H2O2
and Glycine in CMP of Copper Studied with Impedance Spectroscopy", Journal
of The Electrochemical Society 151 (2004) G717-G722.
|