|
Surface
Plasmon Resonance Studies of
Thin-Film Interfaces |
The technique of surface plasmon resonance (SPR) is commonly used in chemical and biological sensors, and different types of SPR sensors are commercially available now. Our SPR studies focus in general on the analysis of various adsorption processes and reaction kinetics at thin film interfaces, where the reactive surface typically represents a chemically functionalized (or unmodified) continuous gold film. The gold-supported interface is often incorporated in a multilayer structure, and sometimes reactions and/or adsorption processes may occur at different regions of the layered interfaces.
Fig. 1 below shows a nanoscale multilayer architecture that we often use for our SPR measurements. The entire structure is supported by a glass slide (of specific optical characteristics, depending on the application), which is about 1 mm thick. On top of it, we have a 35-45 nm thick vacuum-deposited Au layer, which is very smooth, like a single crystal surface. A much thinner layer of Cr is pre-deposited before the Au layer is formed. This Cr acts as a binder layer, and prevents the Au from getting washed off in systems where aqueous solutions are used.
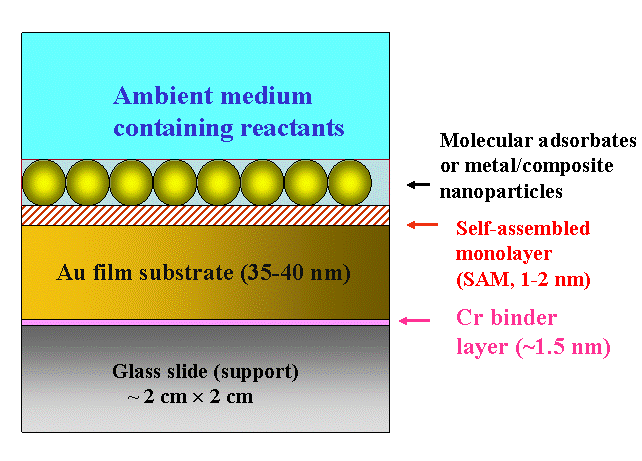
Figure 1. Schematic illustration of a certain type of nanostructured multilayer system studied in our lab using SPR
After thoroughly cleaning the surface of the gold layer, a self assembled molecular monolayer (SAM) is often deposited on the Au. The SAMs typically used in these experiments are alkanethiols with their lengths determined by the number of methylene groups in the hydrocarbon chains. The molecule adsorbs onto the surface with its S-H head group oriented towards the Au substrate (sulfur has a unique tendency for strongly binding to Au). The CH2 groups in the chains of neighboring molecules interact through Van der Waals forces and orient the molecular film in an ordered, compact, and essentially crystalline structure. The stable, chemisorbed molecular layer obtained in this way form a SAM. The surface functionality of the SAM layer can be tailored by appropriately choosing the molecular tail group.

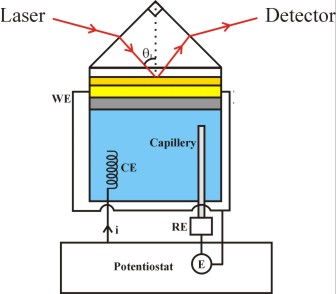
Figure 2. A six-phase multylayer system (not drawn to scale) , incorporated in the Kretschmann configuration of attenuated total reflection (ATR) SPR measurement.
To use the mulilayer structure of Fig. 1 for SPR measurements, the supporting glass slide is optically coupled, by using an appropriate refractive index matching fluid, to a right angled prism of the same glass. This arrangement provides the Kretschmann configuration of ATR for SPR measurement as schematically shown in Fig. 2. The different layers shown in Figure 2 are: (1) an optically transparent SF-10 prism (generally used for studying reactions in aqueous media), (2) a binder layer of Cr (~1.5 nm thick), (3) "inner layer" of the Au SPR substrate (~38-50 nm), (4) surface layer (~ 0.6 nm, electrically charged in electrochemical measurements) of the Au substrate that can act as the sensing element, (5) a "surface sensing element" used for certain applications - typically a self-assembled organic layer (1-3 nm thick), or a metal nanoparticle layer of 10-25 nm thickness, and (6) the ambient medium. The probe laser beam is incident at an angle greater than the critical angle. Surface plasmon waves are generated at the Au surface, and experimental data are recorded in the ATR configuration in the form of the optical reflectivity of the system by varying the angle or the wavelength of optical incidence.
Depending on the parameter (angle or wavelength) varied to generate the
experimental "spectrum", SPR can be classified as an angle resolved
or a wavelength resolved spectroscopy, and our SPR experiments use the
former. As the incident angle (theta) is varied beyond the critical angle
(theta_c) with the incident wavelength kept fixed,, the measured reflectivity
of the multilayer structure goes through a minimum at a certain angle
(usually referred to as the SPR angle, theta_p) where the energy and momentum
of the incident photons match those of the surface plasmon wave. The resulting
graph of reflectivity vs. incidence angle represents what we generally
call an "SPR plot". The overall shape of such an SPR plot is
schematically shown in Fig. 3A below. It should be noted that this specific
type of experiment correspond to the so-called "propagating SPR",
and is different from the case of "localized SPR" observed using
rough structures, island-like films and particles of certain metals (mostly
with Au and Ag in visible and rear-infrared wavelength regions). The differences
between propagating and localized SPR have been discussed in the commonly
available literature, including the review article listed as Ref. [2]
below.
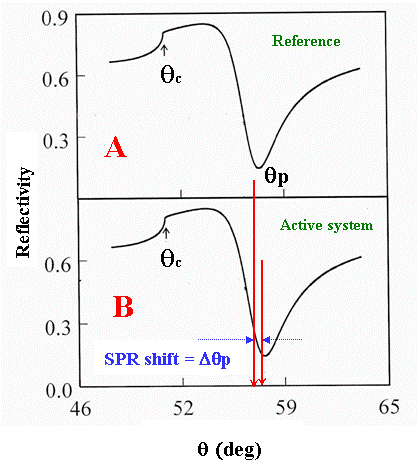
Figure 3. Shift in the SPR angle (at a fixed incident wavelength) as a result of reaction-induced structural/electronic changes of the interfacial regions of a multilayered structure. If the reactions are strictly surface localized and do not affect the bulk solution (phase 6 in Fig. 2), no changes in the critical angle should accompany the SPR angle shift.
Each layer (j) in the multilayer SPR system is optically
characterized by its thickness (dj), refractive indices (nj) and extinction
coefficient (kj). Adsorption and/or reaction induced processes lead to
modifications in the physical properties of the interfaces, which in turn
selectively or collectively change dj, nj and kj for one or more of the
interfaces within the SPR architecture. This results in a shift in the
SPR angle as schematically shown in Fig. 3B.
Using transfer matrix formalism, the SPR angle can be expressed in terms
of the optical parameters of the intermediate layers of the multiphase
system. Thus, the observed shifts in the SPR angle can be calculated by
introducing changes in one or more optical parameters of the multilayer
system. Within the limits of certain approximations, it is also possible
to derive analytical formulas correlating SPR shifts with the interfacial
optical parameters (as shown for instance, in Ref. [7] listed below).
By fitting experimental SPR plots to calculated results, it is possible
to determine the changes in the optical parameters of the constituent
phases of the SPR system. Subsequent analysis of these latter parameters
provide information about the mechanisms and kinetics of the interfacial
processes responsible for the measured SPR angle shifts. We use MATLab
for these calculations, and the necessary software codes have been developed
by our group using commonly known multilayer reflectivity models.
Currently our experiments are focused on developing a systematic expansion of the capability of the SPR technique, by combining time resolved SPR measurements with time resolved Fourier transform electrochemical impedance spectroscopy (FT- EIS). This combination of techniques allows us to perform detailed time resolved kinetic analysis of various complex surface reactions involving nanoscale systems. A typical application of these combined measurements can be found in Ref. [1] listed below.
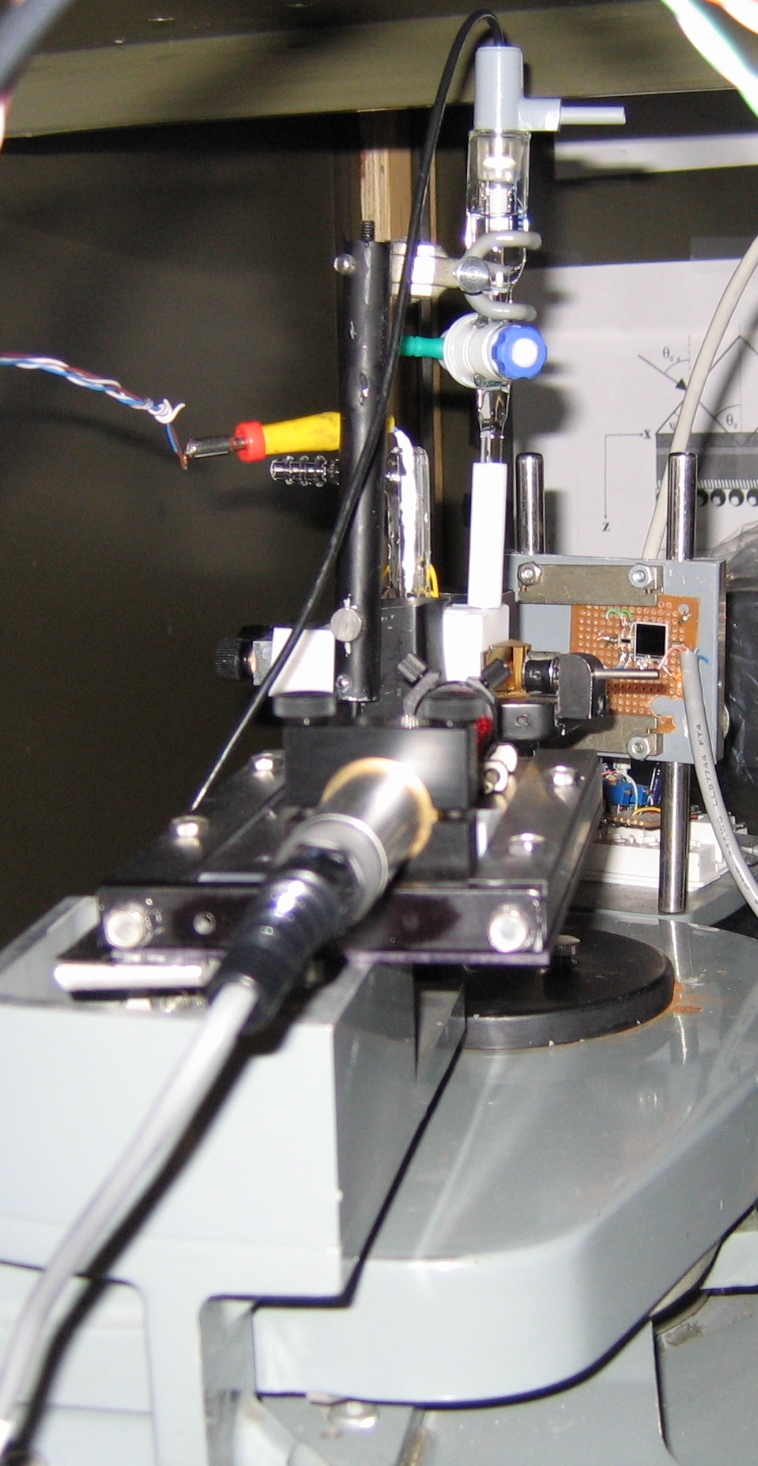
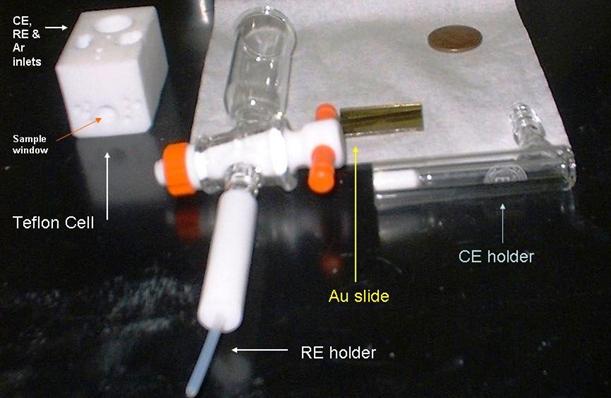
A schematic diagram of the electrochemical SPR cell used in our lab is shown in Figs. 4 and 5. A close-up view of the main components of our SPR setup is shown in Fig.6.
|
|
|
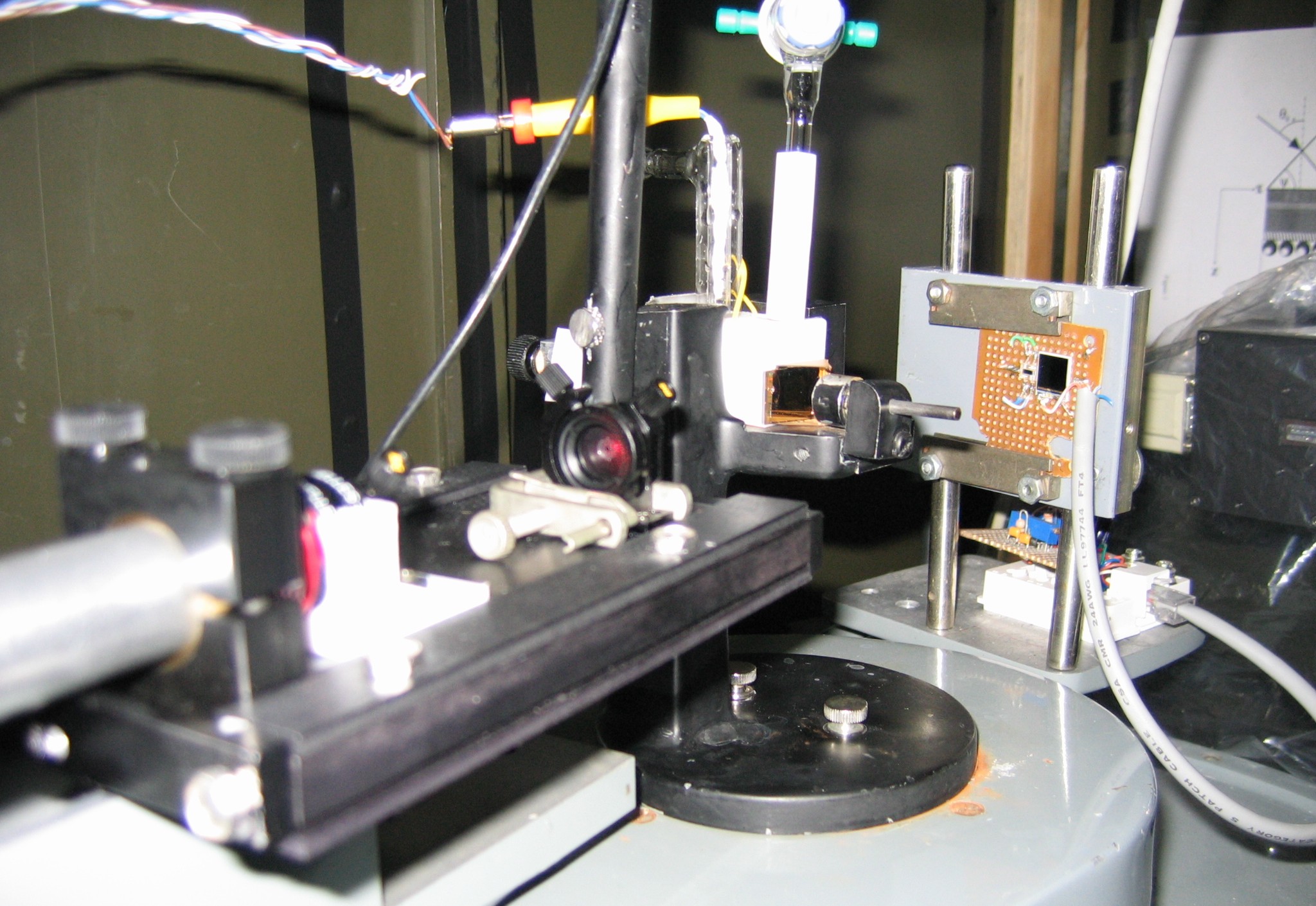
Figure 5. Part of our SPR setup. The sample assembly is arranged on goniometer dial, and the latter is turned at a preset speed. The experiment is controlled using an optical decoder and LabVIEW software. Experimentally, we measure the total angular deviation of the incident beam. Then the angle (theta) of optical incidence inside the prism is calculated in terms of the deviation angle by using Snell's law of refraction. The working principle and other details of this instrument are described in Ref. [3].
Figure 6. A typical electrochemical SPR cell used in our laboratory. The penny is included in the picture to indicate relative dimensions.
Recent Reports of our SPR studies
The direct journal links will only work if your library has subscriptions
[13] C. M. Pettit and D. Roy, "Surface Plasmon Resonance as a Time Resolved Probe of Structural Changes in Molecular Films: Considerations for Correlating Resonance Shifts with Adsorbate Layer Parameters", The Analyst 132 (2007) 524-535.
[12] K.A. Assiongbon and D. Roy, "Electro-oxidation
of methanol on gold in alkaline media: Adsorption characteristics of reaction
intermediates studied using time resolved electro-chemical impedance and
surface plasmon resonance techniques", Surface Science, 594,
[11] D. Roy and J. H. Fendler, "Reflection and Absorption Techniques for Optical Characterization of Chemically Assembled Nanomaterials", Advanced Materials 16 (2004) 479-508.
[10] C. M. Pettit, K. A. Assiongbon, J. E. Garland and D. Roy , "Time Resolved Detection of Electrochemical Effects by Surface Plasmon Resonance Measurements: A Simple Technique Using a Large Area Single Cell Photodiode", Sensors and Actuators B 96 (2003) 105-113.
[9] L.A. Luck, M.J. Moravan, J.E. Garland, B. Salopek-Sondi and D. Roy, "Chemisorptions of Bacterial Receptors for Hydrophobic Amino Acids and Sugars on Gold for Biosensor Applications: A surface Plasmon Resonance Study of Genetically Engineered Proteins", Biosensors and Bioelectronics 19 (2003) 249-259.
[8] J. E. Garland, K. A. Assiongbon, C. M. Pettit and D. Roy, "Surface Plasmon Resonance Transients at an Electrochemical Interface: Time Resolved Measurements Using a Bicell Photodiode", Analytica Chimica Acta 475 (2003) 47-58.
[7] S. Chah, J. Yi, C. M. Pettit, D. Roy, and J. H. Fendler, "Ionization and Reprotonation of Self-Assembled Mercaptopropionic Acid Monolayers Investigated by Surface Plasmon Resonance Measurements", Langmuir 18 (2002) 314-318.
[6] D. Roy, "Optical Characterization of Multi-Layer Thin Films Using the Surface Plasmon Resonance Method: A Six-Phase Model Based on the Kretschmann Formalism", Optics Communications 200 (2001) 119-130.
[5] E. Hutter, J. H. Fendler, and D. Roy, "Surface Plasmon Resonance Studies of Gold and Silver Nanoparticles Linked to Gold and Silver Substrates by 2-Aminoethanethiol and 1,6-Hexanedithiol", Journal of Physical Chemistry B 105 (2001) 11159-11168.
[4] S. Chah, E. Hutter, D. Roy, J. H.Fendler and J. Yi, "The Effect of Substrate Metal on 2-Aminoethanethiol and Nanoparticle Enhanced Surface Plasmon Resonance Imaging", Chemical Physics 272 (2001) 127-136.
[3] D. Roy, "Surface Plasmom Resonance Spectroscopy of Dielectric Coated Gold and Silver Films on Supporting Metal Layers: Reflectivity Formulas in the Kretschmann Formalism", Applied Spectroscopy 55 (2001) 1046-1052.
[2] E. Hutter, J. H. Fendler, and D. Roy, "Surface Plasmon Resonance Method for Probing Interactions in Nanostructures: CdS Nanoparticles Linked to Au and Ag Substrates by Self-Assembled Hexanedithiol and Aminoethanethiol Monolayers", Journal of Applied Physics 90 (2001) 1977-1985.
[1] E. Hutter, S. Cha, J-F. Liu, J. Park, J. Yi, J. H. Fendler, and D. Roy, "Role of Substrate Metal in Gold Nanoparticle Enhanced Surface Plasmon Resonance Imaging", Journal of Physical Chemistry B 105 (2001) 8-12.
Disclaimer
Home || Research || Resume || Publications || Group