Filtering of Nanoparticles
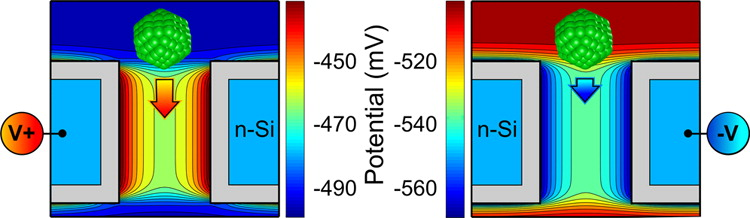
Translocation dynamics of nanoparticles permeating
through the nanopore in an n-Si semiconductor membrane is studied. With
the use of Browninan Dynamics to describe the motion of the charged
nanoparticles in the self-consistent membrnae electrolyte electrostatic
potential, we asses the possibility of using our voltage controlled
membrane ofr the macroscopic filtering of the hcarged nanoparticles.
The results indicate that the tunable local electric field inside the
membrane can effectively control interaction of a nanoparticle with the
nanopore by either blocking its passage or increasing the translocation
rate. The effect is particularly strong for lager nanoparticels due to
their stronger interaction with the membrane while in the nanopore. by
extracting the membrane permeability from our microscopic simulations,
we compute the macroscopic sieving factors and show that hte size
selectivity of the membrane can be tuned by the applied voltage.
Read more...
Slowing down and stretching DNA
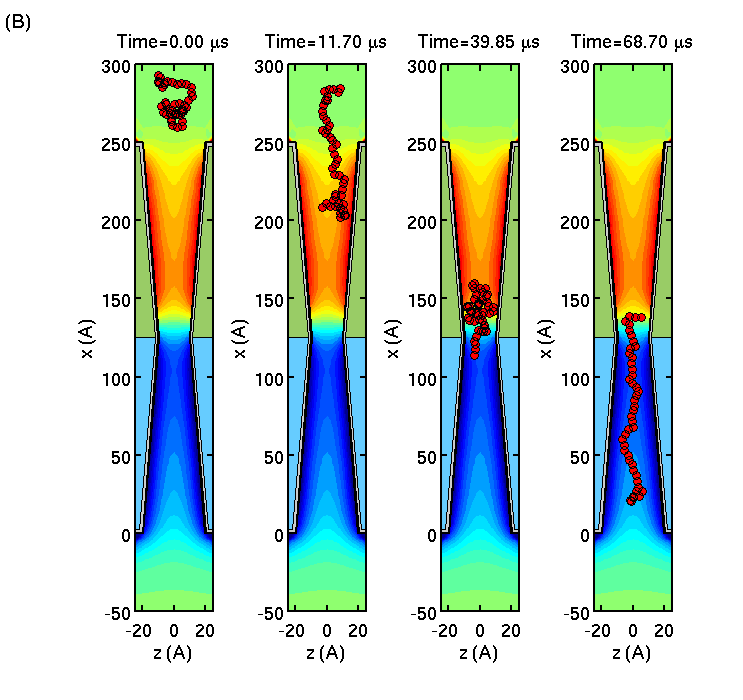
We consider single-stranded DNA translocation through a
semiconductor membrane consisting of doped p- and n-layers of Si forming
a pn-junction. Using Brownian dynamics simulations of the biomolecule
in the self-consistent membrane-electrolyte potential, we show that the
polymer translocation through the membrane is slowed down, while polymer
length is greatly extended.
Read more...
Brownian dynamics of DNA

Numerical simulations can provide us with invaluable
insight into the microscopic behavior of molecules as they translocate
through artificial nanopores. With this in mind, we have developed a
computational tool-box that allows us to examine how polymer dynamics
will be affected by the electrostatic fields of semiconductor membranes
submerged in electrolytic solution.
To simulate the electrostatic potential and the charge carrier
concentrations in the solid-state membrane and the electrolyte, we have
employed the electrostatic approach which is based on the
self-consistent solution of Poisson equation within the semiclassical
approximation for charge carrier statistics in the membrane and
electrolyte.
Read more...
Protein and ion filter

We shown recently that a semiconductor membrane made of
two thin layers of opposite (n- and p-) doping can perform electrically
tunable ion current rectification and filtering in a nanopore. Our model
is based on the solution of the 3D Poisson equation for the
electrostatic potential in a double-cone nanopore combined with a
transport model. It predicts that, for appropriate biasing of the
membrane-electrolyte system, transitions from ohmic behavior to sharp
rectification with vanishing leakage current are achievable.
Furthermore, ion current rectifying and filtering regimes of the
nanopore correspond to different charge states in the p-n membrane,
which can be tuned with appropriate biasing of the n- and p- layers.
Read more...
DNA translocation through a nanopore

We evaluate the magnitude of the electrical signals
produced by DNA translocation through a 1 nm diameter nanopore in a
capacitor membrane with a numerical multi-scale approach, and assess the
possibility of resolving individual nucleotides as well as their types
in the absence of conformational disorder. We show that the maximum
recorded voltage caused by the DNA translocation is about 35 mV, while
the maximum voltage signal due to the DNA backbone is about 30 mV, and
the maximum voltage of a DNA base is about 8 mV. Signals from individual
nucleotides can be identified in the recorded voltage traces,
suggesting a 1 nm diameter pore in a capacitor can be used to accurately
count the number of nucleotides in a DNA strand.
Read more...
Cell biomechanics
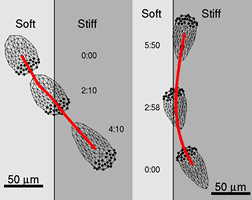
Cell motility is extremely important for many aspects of
life from embryonic development and immunity response to wound healing
and diseases.
It is not fully understood how cells coordinate overall
motility, but from experiments it was established that at least four
different stages of locomotion can be distinguished. Cells crawl by
extending pseudopodia (filopodia or lamellipodia), adhere, contract and
detach the rear. Extension of both filopodia and lamellipodia of most
cells is based on actin polymerization. While the protrusive event of
cell locomotion is thought to be driven by actin polymerization, the
mechanism of forward translocation of the cell body is not completely
understood.
Read more...

